Por que B (CH3) 3 viola a regra do octeto?
#"B(CH"_3)_3# viola o regra do octeto porque o #"B"# atom has only six elétrons de valência in its valence shell.
O regra do octeto is a chemical rule of thumb that states that atoms of main-group elementos combine in such a way that each atom will have eight electrons in its valence shell.
A estrutura de Lewis #"B(CH"_3")"_3# is
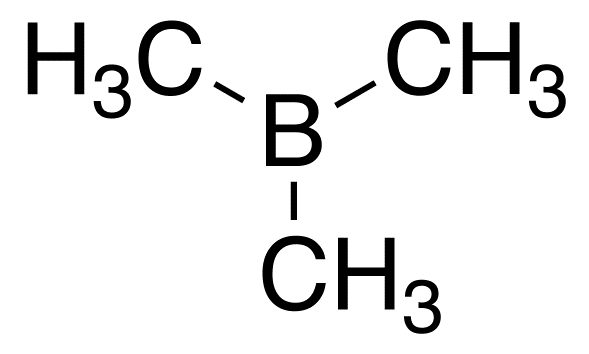
We see that the molecule has only six elétrons de valência em volta do #"B"# átomo.
This is a violation of the octet rule.

