What are the bond angles in the central atom of the following:
#"NSF"#,
#"OF"_2#,
and #"IBr"_2^−"#?
Responda:
The bond angles are slightly less than 120°, slightly less than 109.5 °, and 180 °, respectively.
Explicação:
#bb"NSF"#
A estrutura de #"NSF"# is
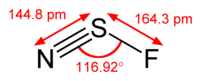
There is a lone pair on the #"N"# atom, a lone pair on the #"S"# atom, and there are three lone pairs on the #"F"# átomo.
Esta é uma #"AX"_2"E"# molecule, so the electron geometry is trigonal planar and the molecular shape is bent.
The theoretical bond angle is 120 °, but repulsion by the lone pairs decreases the bond angle to about 117 °.
#bb"OF"_2#
A estrutura de Lewis #"OF"_2# is

Esta é uma #"AX"_2"E"_2# molecule, so the electron geometry is tetrahedral and the molecular shape is bent.
The theoretical bond angle is 109.5 °, but repulsions by the lone pairs decrease the bond angle to about 103 °.

#bb"IBr"_2^-#
A estrutura de Lewis #"IBr"_2^-# is
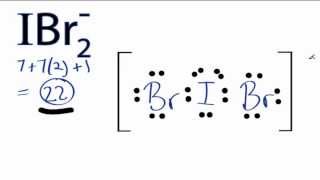
Esta é uma #"AX"_2"E"_3# structure, so the electron geometry is trigonal bipyramidal.

The lone pairs occupy the equatorial positions, with the #"Br"# atoms in the axial locations.
The bond angle is 180 °.

